Oovacha uses AI to reduce the burden on clinical research teams & unlock key trial data and insights




Our flagship platform Reveal Clinical helps clinical research teams drive efficiency and intelligence into critical workflows, reducing the burden of highly manual work and shortening study cycle times


Decrease in cycle time between data entry and query issuance
Man hours saved during the data review process
Increase in data quality check coverage




Yes, Reveal has many pre-built connectors that plug into popular EDC systems (e.g., Medidata Rave, Oracle Clinical One, Merative Zelta). If your study's EDC system is not in our list of pre-built connectors, our team will set a new one up.
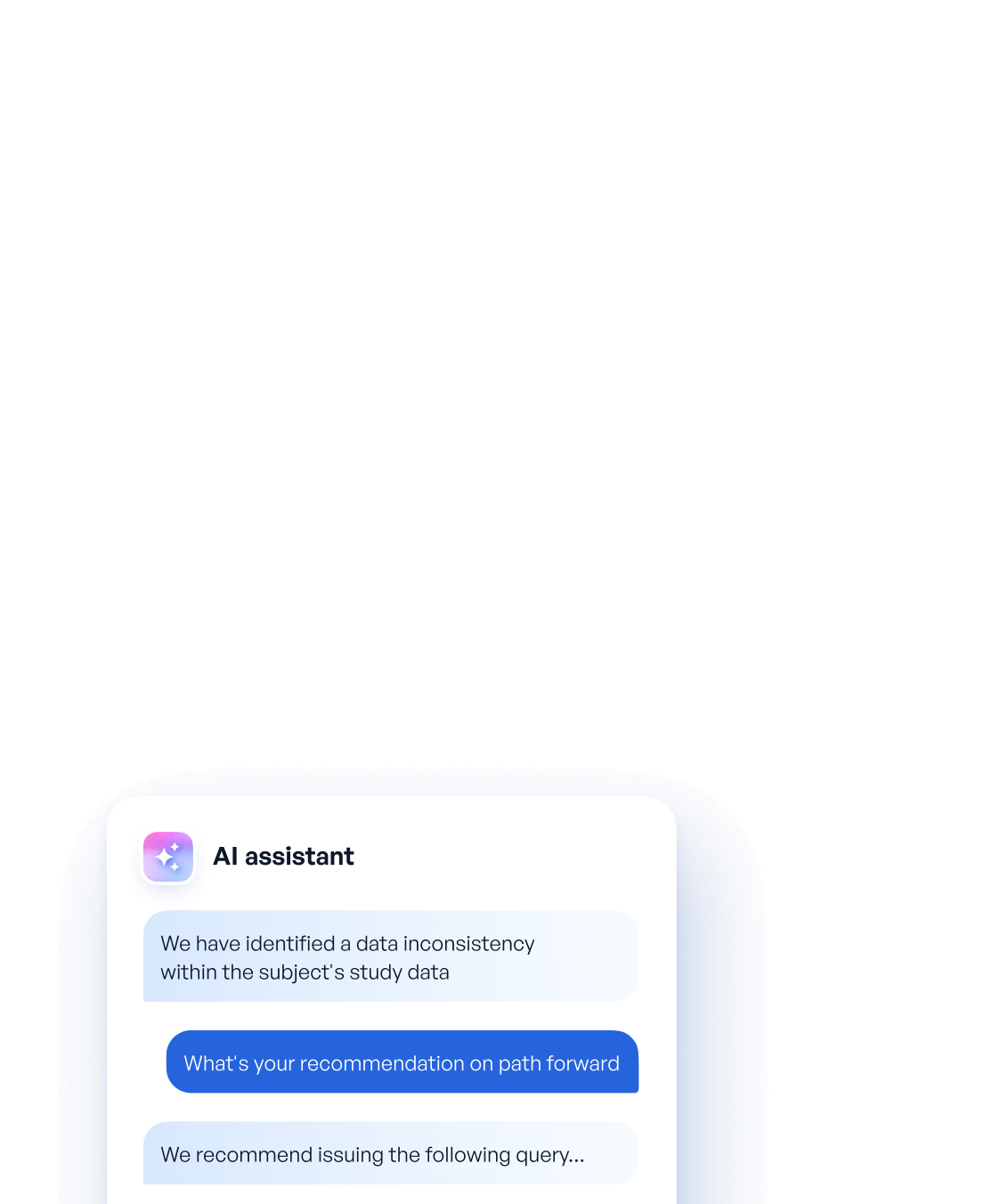
Today, Reveal primarily support clinical data managers as they perform clinical data review and query management during a study
Current data review practices (EDC edit checks + SAS listings) fall short of providing sufficient data quality check coverage. They struggle with identifying errors that involve study/clinical context, textual information, complex cross-form relationships, longitudinal trends or data heterogeneity. Modern AI tools and Reveal excel at identifying these types of errors.
Reveal is meant to be used during study execution, following the EDC build process and prior to Database Lock.
No, Reveal's AI assistants perform analyses and recommend suggestions on their own, but they never take any action or push data to the EDC system without explicit confirmation from users.
Yes, Reveal follows all necessary security and compliance standards such as HIPAA and 21 CFR Part 11. Reveal is also a validated computer system and has a robust suite of validation tests that demonstrate system performance.
Reveal adheres to extremely strict data privacy and governance policies. Data shared with Reveal is never shared with any external parties (including AI model providers) and is governed internally based on the principle of least privileged access.